- Address: Lot No. 20, Zone G, D1 Street, An Ha Industrial Park, Tan Vinh Loc Commune, Ho Chi Minh City
- Phone: (84-28) 668.36156 / 668.36158
- Hotline: 1800.9435
- Fax: (84-28) 3620.4694
- Email: vibo@vibo.com.vn
- Website https://vibo.com.vn/

PREVENTION AND TREATMENT SOLUTIONS FOR TRANSLUCENT POST-LARVAE DISEASE (TPD) IN SHRIMP
A. TRANSLUCENT POST-LARVAE DISEASE (TPD) – A Major Emerging Threat in Shrimp Farming
1. Current status
Translucent Post-Larvae Disease (TPD), also known as “Glass Post-Larvae Disease,” is an emerging disease affecting Penaeus vannamei. It was first reported in March 2020 in China and is characterized by extremely high mortality rates, particularly in shrimp at the PL4–PL7 stages.
The disease is caused by a highly virulent strain of Vibrio parahaemolyticus, designated VpTPD, which has resulted in severe economic losses—impacting 70–80% of hatcheries as well as shrimp farmers. The disease spreads rapidly and can cause up to 90% mortality within 2–3 days, leading to catastrophic financial losses if not promptly managed.

2. Etiology of TPD
Research has identified that the causative agent is a novel, highly virulent Vibrio parahaemolyticus strain (Vp-JS20200428004-2).
This bacterium carries a plasmid-encoded virulence factor—Vibrio High Virulent Protein-2 (VHVP-2), a subunit of the Tc (toxin complex) toxin system, which is the primary virulence determinant of TPD.
LC-MS/MS analysis shows that this toxin fraction includes Tc toxin subunits:
-
TcA (~300 kDa)
-
TcB (~200 kDa)
-
TcC (~100 kDa)
VHVP-1 and VHVP-2 correspond to tccC1 and tccC2, encoding TcC toxin subunits that determine toxin specificity (Shuang Liu et al.).
The Tc toxin complex is commonly found in entomopathogenic bacteria such as Photorhabdus and Xenorhabdus, but has now been identified in V. parahaemolyticus (Kathy F.J. Tang, Donald V. Lightner et al.).
Further studies have revealed that other Vibrio species, such as V. harveyi and V. campbellii, may also carry the VHVP-2 gene, suggesting a diverse range of potential TPD pathogens.
B. DIAGNOSTIC METHODS
Early detection of TPD is critical for effective control and mitigation. The following are the primary diagnostic approaches used to identify Translucent Post-Larvae Disease.



1. Clinical Symptom-Based Diagnosis
-
Early stage (6–12 hours post-infection):
✔️ Reduced or complete loss of feeding.
✔️ Lethargic swimming and weak reflexes.
✔️ Gills appear pale yellow—an early indicative sign.


-
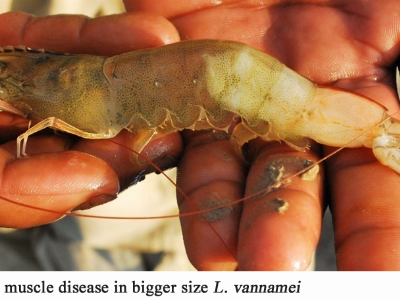
After 24–48 hours: (Figure 2)
✔️ Hepatopancreas color changes from dark brown to light brown.
✔️ Intestine becomes transparent like glass; empty stomach.
✔️ Gills swollen, loose, and easily damaged.
✔️ Hepatopancreas showing necrosis and pallor, reduced digestive function.
✔️ Body becomes whitish and translucent; muscle atrophy observed.
✔️ Rapid weakening followed by mass mortality if untreated.
2. Histopathological Diagnosis
Microscopic examination reveals:
-
Degeneration, necrosis, and loss of epithelial integrity in the hepatopancreatic tubules and midgut.
-
Characteristic sign: rapid disintegration of hepatopancreatic cells with numerous necrotic foci.
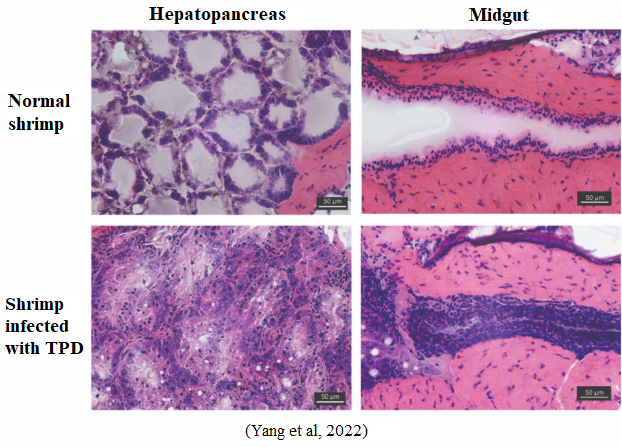
Figure 3: Histopathological comparison between normal shrimp and TPD-infected shrimp (hepatopancreas and midgut).
3. Molecular Diagnosis (PCR / qPCR)
Genetic detection of the causative pathogen.
PCR or qPCR is used to detect vhvp-2 and vhvp-1 genes in Vibrio parahaemolyticus—the primary agent causing TPD.
-
Advantages: High accuracy, able to detect infection even before visible symptoms.
-
Limitations: Requires laboratory facilities and trained personnel.
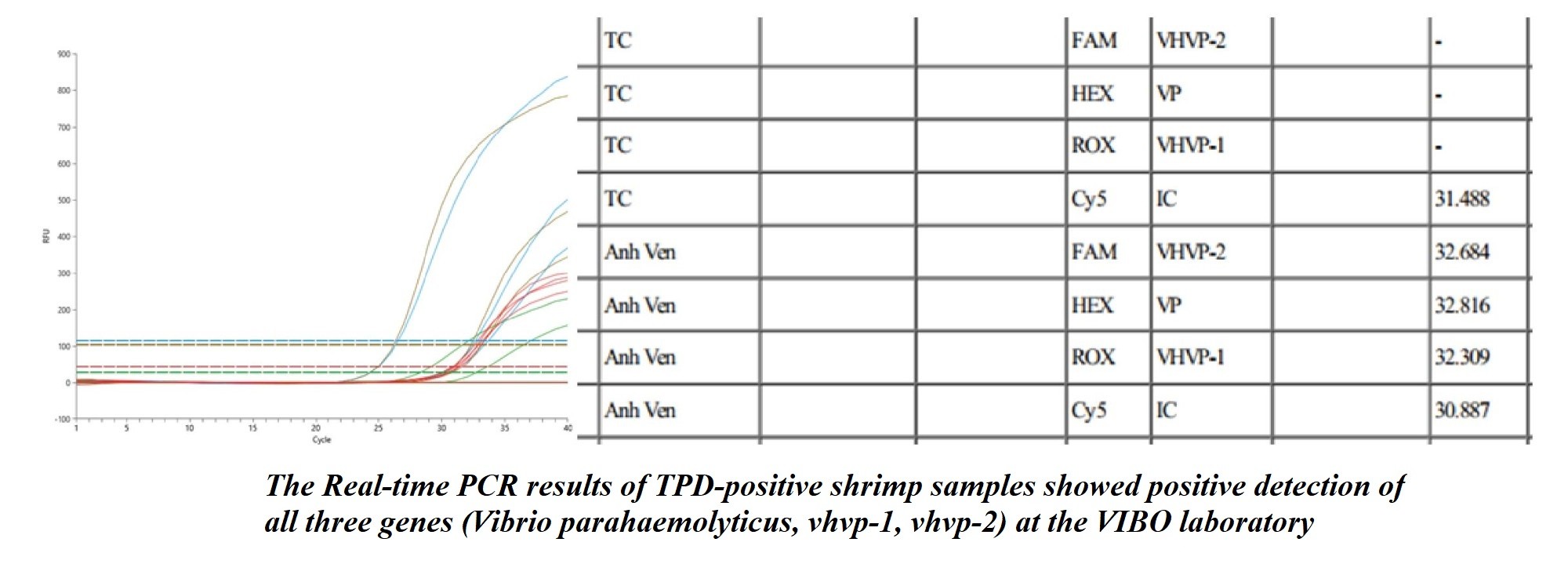
Figure 4: Real-time PCR results showing positive amplification of three genes (V. parahaemolyticus, vhvp-1, vhvp-2) for TPD-positive samples at VIBO Laboratory.
4. Microbiological Diagnosis
Bacterial isolation from infected samples:
-
Collect hepatopancreas, intestine, and pond water samples.
-
Culture on TCBS or CHROMagar Vibrio.
-
Green colonies on TCBS or purple colonies on CHROMagar indicate Vibrio.
-
-
Perform biochemical tests (Gram stain, oxidase, catalase) to confirm V. parahaemolyticus.
-
Advantages: Confirms specific bacterial strains.
-
Limitations: Requires 24–48 hours; not ideal for urgent intervention.
C. PREVENTION AND TREATMENT PROTOCOLS FOR TPD IN POST-LARVAE AND NEWLY STOCKED SHRIMP
3.1. Preventive Protocol
Active disease prevention should begin from the earliest stage by enhancing natural immunity, enabling shrimp to proactively resist pathogenic bacteria. This should be combined with stabilizing the digestive system and hepatopancreas to strengthen overall resistance and reduce the risk of Vibrio infection. Concurrently, maintaining strict environmental control and stable water quality helps limit the proliferation of pathogenic bacteria. Supplementing antagonistic probiotics further suppresses Vibrio, reducing the likelihood of disease outbreaks.
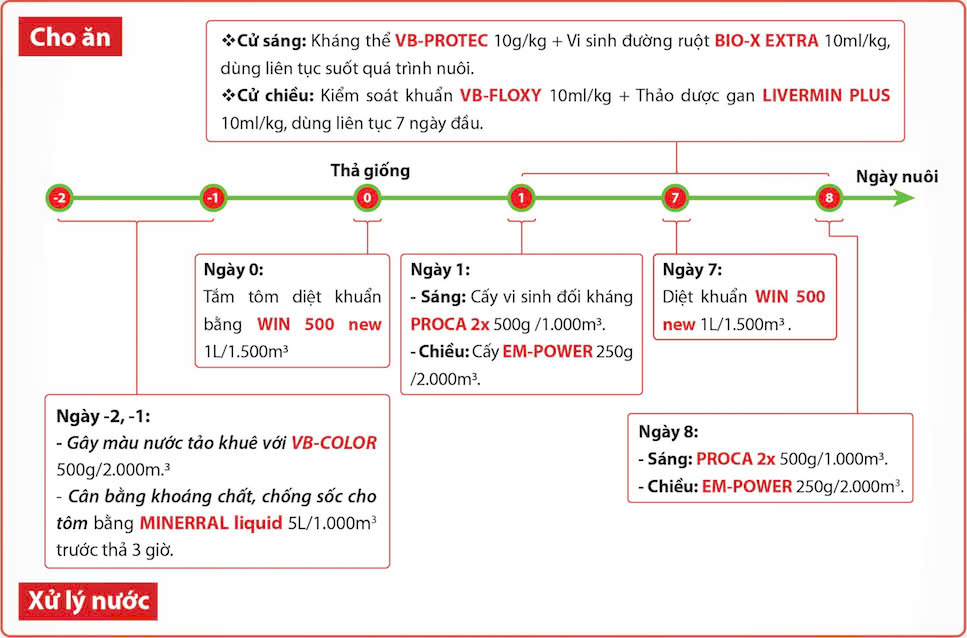
Apply Vibo’s biological product line to achieve the highest level of preventive effectiveness:
Feeding Program
Morning Feeding:
-
Antibody supplement VB-PROTEC at 10 g/kg feed
- Gut probiotic BIO-X EXTRA at 10 ml/kg feed, used continuously throughout the culture period.
Afternoon Feeding:
-
Bacterial control with VB-FLOXY at 10 ml/kg feed
- Herbal hepatopancreas tonic LIVERMN PLUS at 10 ml/kg feed, used continuously for the first 7 days.
Stocking Schedule
Day –2 and –1 (Before Stocking):
-
Enhance water color with VB-COLOR at 500 g/2,000 m³.
-
Balance minerals and reduce stress for shrimp using MINNERAL Liquid at 5 L/1,000 m³, applied 3 hours before stocking.
Day 0: (Stocking Day)
-
Disinfect shrimp by bathing them in WIN 500 new at 1 L/1,500 m³.
Day 1:
-
Morning: Apply antagonistic probiotics PROCA 2x at 500 g/1,000 m³.
-
Afternoon: Apply EM-POWER at 250 g/2,000 m³.
Day 7:
-
Disinfect the pond with WIN 500 new at 1 L/1,500 m³.
Day 8:
-
Morning: Apply PROCA 2x at 500 g/1,000 m³.
-
Afternoon: Apply EM-POWER at 250 g/2,000 m³.
3.2. Treatment Protocol
Treatment Protocol When Shrimp Are Infected:
✔ Withhold feed for one day to reduce digestive stress and allow shrimp to recover.
✔ Apply pond-water disinfection to control Vibrio directly from the external environment.
✔ Combine antagonistic probiotics to suppress Vibrio, prevent reinfection, and support faster recovery.
✔ Supplement hepatopancreas- and digestive-support compounds to reduce the impact of toxins secreted by Vibrio.
✔ Use appropriate antibiotics to control intestinal bacteria, ensuring no drug resistance occurs while helping shrimp recover quickly.
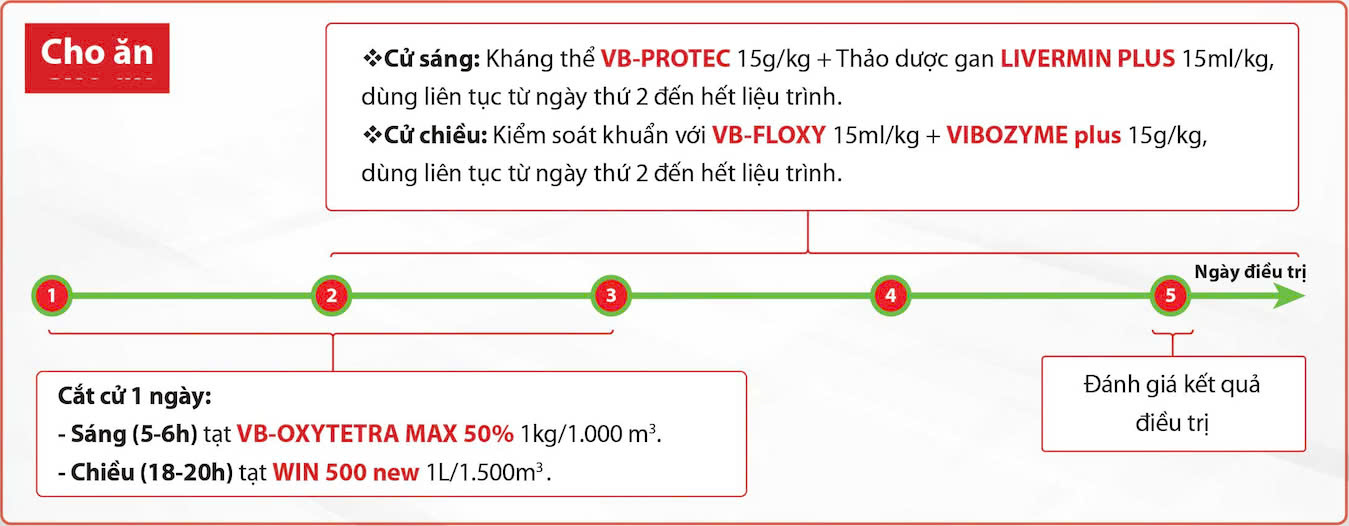
Feeding Program
Morning Feeding:
-
Antibody supplement VB-PROTEC at 15 g/kg feed
- Hepatopancreas herbal tonic LIVERMN PLUS at 15 ml/kg, used continuously from Day 2 until the end of the treatment course.
Afternoon Feeding:
-
Bacterial control with VB-FLOXY at 15 ml/kg feed
- Digestive enzyme VIBOZYME plus at 15 g/kg, used continuously from Day 2 until the end of the treatment course.
Treatment Timeline
Day 1 — Withhold feed for 1 day:
-
Morning (5–6 AM): Apply VB-OXYTETRA MAX 50% at 1 kg/1,000 m³.
-
Afternoon (18:00–20:00): Apply WIN 500 new at 1 L/1,500 m³.
Days 2 → 5:
Continue the feeding and pond-treatment steps as shown in the timeline.
Day 5 — Treatment Outcome Evaluation
Evaluate the results of the treatment and adjust protocols if necessary.
Specialized shrimp article by the VIBO Technical Department