- Address: Lot No. 20, Zone G, D1 Street, An Ha Industrial Park, Tan Vinh Loc Commune, Ho Chi Minh City
- Phone: (84-28) 668.36156 / 668.36158
- Hotline: 1800.9435
- Fax: (84-28) 3620.4694
- Email: vibo@vibo.com.vn
- Website https://vibo.com.vn/

B. subtilis DSM33018 is able to degrade pirB and alleviates AHPND in Artemia
B. subtilis DSM33018 is able to degrade pirB and alleviates AHPND in Artemia

Early mortality syndrome (EMS) or acute hepatopancreatic necrosis disease (AHPND), is a penaeid shrimp disease that causes serious economic losses and significant mortality, up to 100%, in cultured shrimp species.
The dramatically high mortality rates in infected shrimp are caused by dysfunction and destruction of the hepatopancreas (Lightner et al. 2014). There is no inflammatory response to the causative Vibrio spp., because AHPND is elicited by a toxin (Han et al. 2015), which is encoded by a plasmid (Yang et al. 2013; Tran et al. 2013). Various Vibrio spp., not only V. parahaemolyticus were demonstrated to carry this pathogenic plasmid.
Comparison of genome sequences revealed that the pathogenic plasmid encodes genes homologous with Photorhabdus insect-related (Pir) toxin genes (Kondo et al. 2014). The Pir toxins act as binary proteins, they are encoded by the pirA and pirB genes, and both proteins are necessary for oral toxicity (Blackburn et al. 2006; Ahantarig et al. 2009; Han et al. 2015).
At the Laboratory of Aquaculture and Artemia Reference Center of the University of Ghent, it was tested, if B. subtilis DSM 33018 is able to degrade the AHPND-causing pirB toxin in vitro. It could indeed be demonstrated that B. subtilis DSM 33018 is able to degrade the AHPND-causing pirB toxin (Figure 1).
Gram-positive probiotic organisms, such as Bacillus subtilis from Biomin are not involved in horizontal gene transfer processes with Gram-negative organisms such as Vibrio spp. and are thus unlikely to acquire resistance or virulence genes or plasmids from Vibrio species (Moriarty, 1999). B. subtilis DSM 33018 is part of the probiotic product range AquaStar® with scientifically proven positive effects on aquaculture productivity.
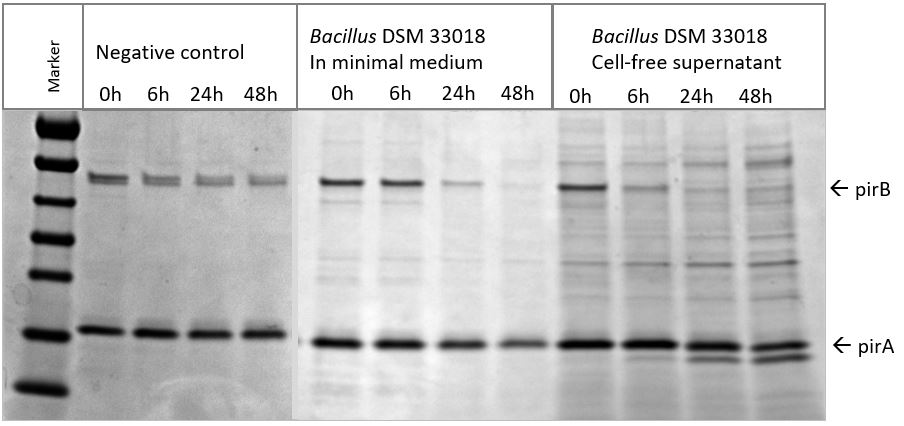
Figure 1: Bacillus subtilis DSM33018 is able to degrade pirB, one of the two subunit toxins needed to elicit AHPND in shrimp. SDS page was performed to visualize pirB degradation by B. subtilis DSM33018 cells and cell-free supernatant. The darker a band is on this SDS page gel, the more protein (pirB or pirA) is present.
It was then tested if B. subtilis DSM33018 is able to rescue infected animals from AHPND related mortality in gnotobiotic artemia. Bacillus subtilis DSM 33018 (Ba) was tested at 107cfu/mL and 108 CFU/mL. At the concentration of 107 CFU/mL, Bacillus treatment resulted in a survival rate of 63% versus 47% in control (Figure 2). Enterococcus (En), Pediococcus (Pe), and Lactobacillus (La) alone were not able to significantly improve the survival rate after the toxin challenge. AquaStar® (Mix) at concentrations 107 CFU/mL and 108 CFU/mL however rescued the artemia from AHPND related mortality and allowed survival rates of 77% and 70%.
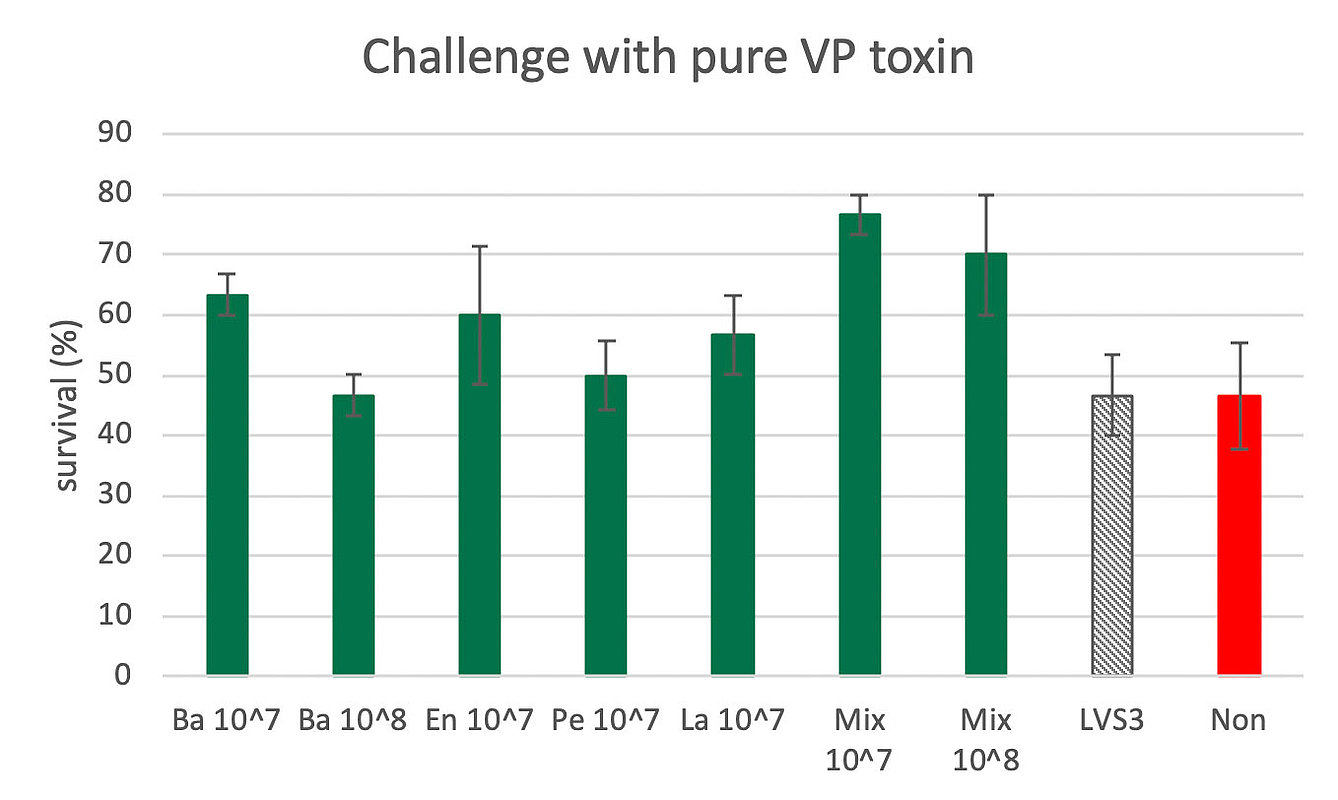
Figure 2. Artemia survival after challenge with purified pir toxins at 26 µg/mL. Bacillus DSM33018 (Ba) was tested at 107cfu/mL and 108 CFU/mL. At the concentration of 107 CFU/mL, it tended to increase the survival rate from 47% in control to 63%. Enterococcus (En), Pediococcus (Pe), and Lactobacillus (La) alone tended to improve the survival rate after the challenge. AquaStar® (Mix) at concentrations 107 CFU/mL and 108 CFU/mL rescued the artemia from AHPND related mortality and allowed survival rates of 77% and 70%. LVS3 is a sterile Aeromonas control, acting as nutritional support. ‘Non’ is the control challenge without probiotic treatment nor basic nutritional support.
It remains to be determined if B. subtilis alone helps to alleviate AHPND in shrimp culture. Having said that, when Pacific white leg shrimp (L. vannamei) were challenged with pirA and pirB toxin-producing Vibrio parahaemolyticus (AHPND positive), via an immersion infection model, supplementing AquaStar® Growout (a combination of Bacillus sp. plus lactic acid bacteria) appeared to significantly reduce mortalities (Figure 3; Kesselring et al, 2019).
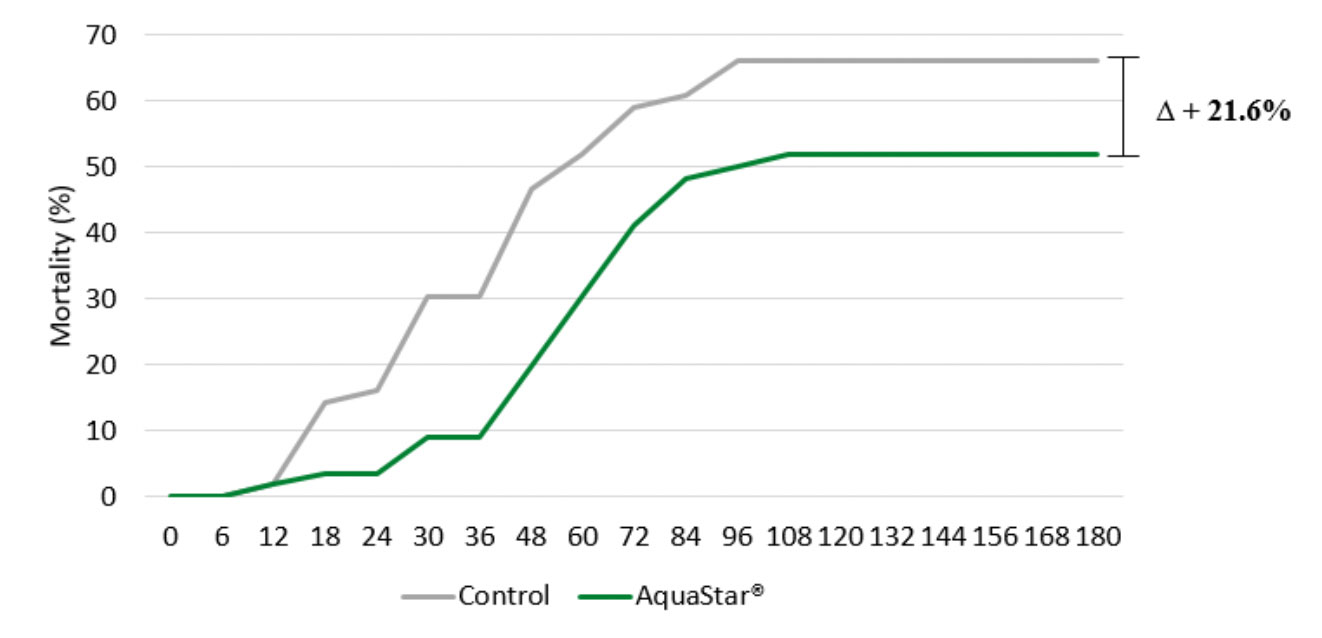
Figure 3. Mortality of shrimp fed control (grey) or AquaStar® Growout (green) diets after a V. parahaemolyticus (AHPND positive) immersion challenge.
Vibo Technical Department